
As the pandemic continues, strategies and policies for vaccine distribution and administration are urgently needed. Sustained leadership and execution from the federal government is critical for moving forward to best address the pandemic and ensure that health information and technology is being properly utilized. Maximizing the role of health IT in evolving strategies in development, and the value provided by health professionals in tackling COVID-19, can be critical—particularly surrounding accelerating vaccination distribution and administration.
Ensuring a data-driven response to COVID-19 and future high-consequence public health threats, as well as the national strategy for the COVID-19 response and pandemic preparedness are two examples where federal direction has already been helpful to state, territorial, local and tribal governments on how best to proceed.
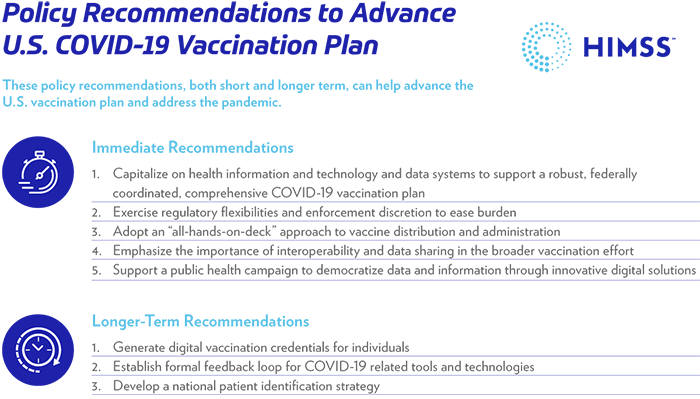
The federal government has an opportunity to build on existing efforts. For example, health information and technology should play an important role in the plans identified by the COVID-19 Health Equity Task Force. Improved and consistent data reporting will help identify gaps in vaccination distribution and drive targeted approaches for underserved populations. More reporting guidance from the federal government can help set clearer expectations and track whether vaccine doses are making it to the populations that are in most need.
COVID-19 has underscored the critical need for a large-scale upgrade to public health data infrastructure. The U.S. federal government should integrate any approach to managing immunizations into a broader infrastructure that optimizes data collection and state/local health department access to data to manage ongoing and emergent health crises. The investment in Centers for Disease Control and Prevention’s Data Modernization Initiative thus far has been critical, however long-term funding is essential to modernize and maintain public health surveillance.
Another example of how the federal government can support vaccine distribution and administration is to ease administrative and clinician burden by broadening regulatory flexibilities and enforcement discretion. The Health and Human Services Office of Civil Rights’ announcement on enforcement discretion for use by certain providers of web-based scheduling applications for COVID-19 vaccination appointments is a positive step. The government should also explore the continued and additional temporary use of regulatory flexibilities to reduce burden, while creating a robust preparedness program that utilizes health information and technology that is fully conformant under HIPAA.
An “all-hands-on-deck” approach is essential and a federally coordinated response can strengthen connections. Essential stakeholders include EHR market suppliers, pharmacies and other vaccination providers, public health departments and immunization information systems, HIEs, health plans, health systems, and non-governmental organizations, such as the World Health Organization.
Recognizing the need for a federally coordinated government approach to solving these issues, HIMSS and our partners have offered the U.S. government recommendations on how to improve COVID-19 vaccine distribution and administration by maximizing the use of information and technology and are offering to help support these efforts.
COVID-19 Global Policy Call to Action
HIMSS calls on government, businesses, civil society leaders and elected officials to recognize the important role and value of health information and technology during a health emergency and to work across industries to leverage sound health data, tools of informatics and innovative solutions outlined in our Global Policy Call to Action.